Mastering Immune Complexity
1/2/20254 min read
Unlocking the Power of Diversity in the Immune System
The human immune system is a testament to the diversity that defines our species. This diversity is our strength, enabling us to survive countless threats. However, it also presents a unique challenge for modern medicine: developing therapies that are robust enough to work across all individuals. The heterogeneity of immune responses complicates the discovery of universal treatments, making drug development a complex and intricate endeavor.
The Complexity of Studying the Immune System
Understanding the immune system requires navigating its immense complexity. We often resort to reductionist approaches, isolating specific components to study their roles. While this simplifies the system and yields insights, it sacrifices the broader context, omitting the intricate interactions within the system. As a result, much of the immune system’s complexity—and its potential—is left untapped.
Why Modern Tools Aren’t Enough
Advancements like computational models for predicting therapeutic structures have revolutionized drug discovery. However, these tools fail to address a critical gap: translating disease understanding into real-world, effective drugs. Determining whether a drug candidate is both safe and efficacious remains the most expensive and time-consuming part of the drug discovery process. This inefficiency drives up the cost of developing new medicines, leaving many potential therapies unrealized.
The Dream of Simulating Biology
For decades, researchers have envisioned a future where complex biological systems could be simulated within a computer. Such simulations would allow scientists to test disease hypotheses, design treatments, anticipate side effects, and even personalize therapies. Yet, the immune system’s intricate and dynamic nature has made this dream nearly impossible, as existing models of the immune system often lack the accuracy to deliver actionable predictions.
AnuBio’s Game-Changing Approach
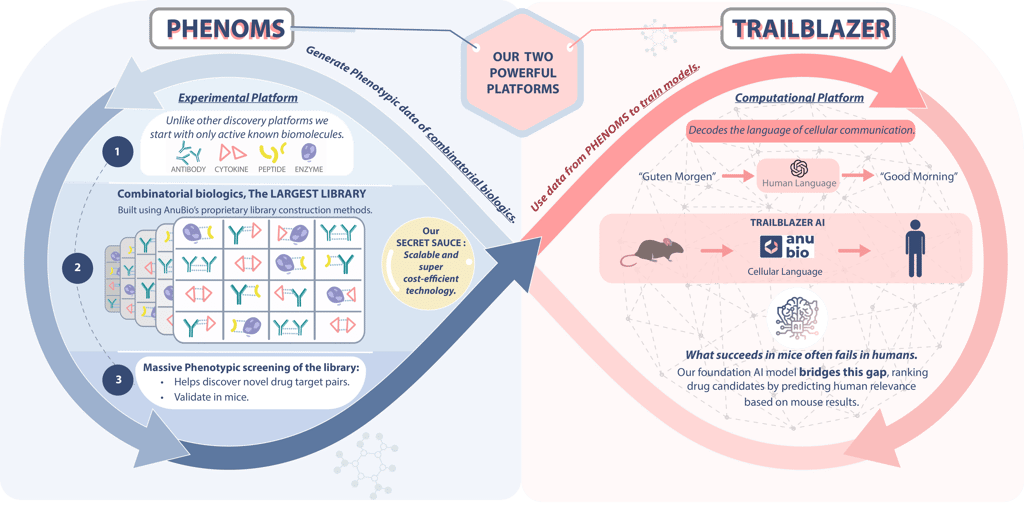
AnuBio is transforming this dream into reality with its innovative closed-loop lab-AI system. This system unites two groundbreaking technologies: PHENOMS and TRAILBLAZER.
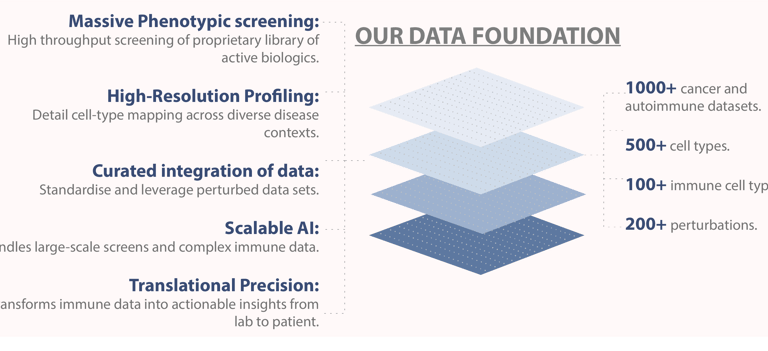
PHENOMS is a proprietary wet lab discovery platform designed for rapid and cost-effective training and validation of AI model predictions. PHENOMS utilizes novel strategy for building combinatorial drugs from simpler immunomodulatory blocks and testing them directly on patient-derived tissues using phenotypic assays.
TRAILBLAZER, on the other hand, is the first generative large multicellular language model capable of accurately capturing and predicting complex cell system behaviors across diverse populations. Together, these technologies create a feedback loop that bridges the gap between theoretical predictions and experimental validation.
AnuBio combines proprietary phenotypic screening platform for combinatorial biologics - PHENOMS with first generative large multicellular language model capable of accurately capturing and predicting complex cell system behaviors across diverse populations.
How TRAILBLAZER sees the Immune System
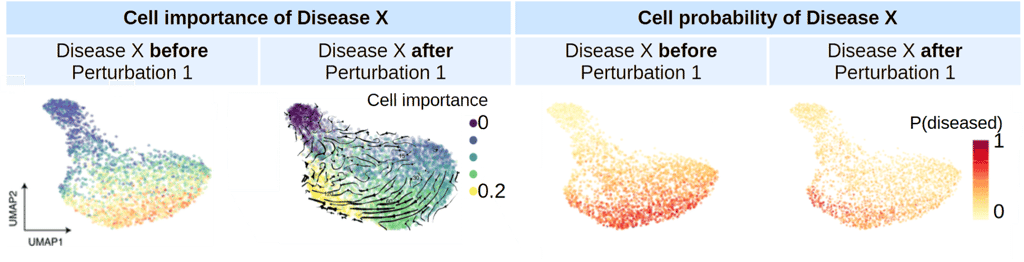
TRAILBLAZER is trained on an extensive dataset of single-cell transcriptomics, encompassing immune diseases, tumor microenvironments, immune states, and a comprehensive library of known immunomodulators. A significant breakthrough is TRAILBLAZER's novel architecture, which reconstructs multicellular states and responses across multiple individuals. This enables the model to predict cell states after virtual treatment with unparalleled accuracy. Additionally, TRAILBLAZER serves as a hypothesis generator, developing an internal understanding of disease mechanisms, identifying potential side effects, and providing actionable insights, thereby paving the way for safer and more effective therapies.

A Case Study: Enhancing Anti-PD1 Responses
TRAILBLAZER’s potential was demonstrated in a study aimed at improving anti-PD1 responses for metastatic breast cancer patients. The model was given two datasets: immune profiles of untreated patients and profiles of patients who responded well to anti-PD1 therapy. With this data, TRAILBLAZER identified combinations of immunomodulators that can shift the immune system from the initial to the desired state. The result was a ranked list of promising therapies, offering researchers a clear path for further investigation.
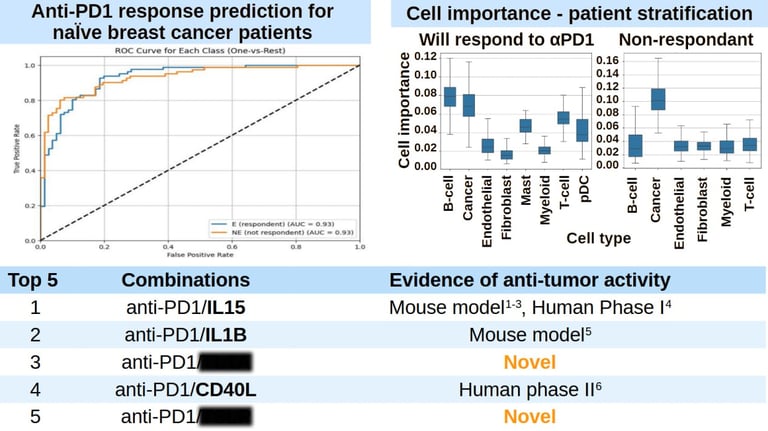
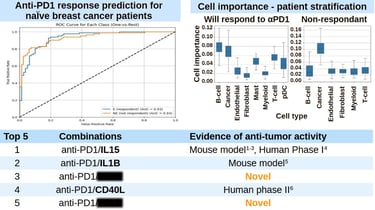
TRAILBLAZER AI accurately stratifies naive breast cancer patients based on their sensitivity to anti-PD1 therapy. It uses its understanding of what makes patient responding to anti-PD1 therapy to find combinations of therapeutics that would improve immune response to cancer cells. Top 5 predictions of the model and their experimental support are listed below. Citations: 1, 2, 3, 4, 5, 6
A Vision for Personalized Medicine
This example highlights the unique strength of AnuBio’s approach. By combining TRAILBLAZER’s predictive power with PHENOMS’ experimental rigor, AnuBio can accelerate the discovery process, reduce costs, and identify therapies for patients resistant to current treatments. This integration ensures that only the most promising candidates move forward, maximizing efficiency and impact.
AnuBio’s vision is more than technological innovation—it’s a revolution in how we approach medicine. By addressing the immune system’s complexity and diversity, PHENOMS and TRAILBLAZER enable a future where therapies are not only effective but also personalized to individual needs. The long-standing dream of simulating biological systems to transform healthcare is becoming a reality, and AnuBio stands at the forefront of this transformation.
****
TRAILBLAZER learns to understand importance of each cell to a disease and to find pharmacological interventions that move the cell system from sick state to healthy state for each patient.
